Table of Contents
- Deep Dive IBDP Physics: - B.1 Thermal Energy Transfers Teacher Resources Pack
- 🌡️ Activity 1: Comparing Convection and Conduction Mechanisms
- 🧪 Activity 2: Applying the Kinetic Theory to Explain Pressure and Temperature in Gases
- 🧠 Synthesis: Building Conceptual Bridges Between Heat and Motion
- 🔥 Turn Up the Engagement in Thermal Physics
Deep Dive IBDP Physics: - B.1 Thermal Energy Transfers Teacher Resources Pack
In the ever-evolving journey of physics education, thermal energy continues to play a pivotal role in helping students understand the interactions of particles, energy, and matter. Within the IB Diploma Programme (IBDP) Physics curriculum, two concepts stand out as essential for learners: heat transfer mechanisms and the kinetic theory of gases.
To empower educators with engaging and rigorous instructional tools, we’re spotlighting two of our most interactive and curriculum-aligned resources:
Comparing Convection and Conduction Mechanisms
Applying the Kinetic Theory to Explain Pressure and Temperature in Gases
These activities transform textbook principles into practical, hands-on learning, strengthening conceptual understanding and supporting skill development in data analysis, experimentation, and critical thinking.
🌡️ Activity 1: Comparing Convection and Conduction Mechanisms
Curriculum Alignment:
IBDP Physics Topic B.1 – Thermal Energy Transfer
Covers heat flow, particle theory, and real-world applications
Learning Outcomes:
Distinguish between conduction and convection as heat transfer methods
Analyze how material type and state affect energy flow
Apply quantitative methods to calculate heat transfer efficiency
Draw conclusions about which method is more effective under different conditions
Activity Overview:
Students perform parallel experiments—one with a metal rod and another with a fluid medium like water or oil—to observe how heat propagates differently through solids and fluids.
Key Equipment:
Metal rod, thermometer, stopwatch, heat source
Beaker of water/oil for convection
Data recording sheet for temperature profiles
Method Highlights:
Record temperature changes over time along the metal rod to observe conduction
Monitor fluid temperature gradients and convection currents visually
Compare the rate and uniformity of heat distribution between the two systems
Theory Involved:
Conduction: Energy moves through direct molecular collisions in solids
Convection: Heated particles in fluids move due to density differences, creating convection currents
Apply formulas:
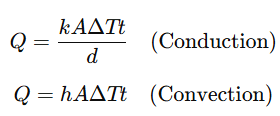
Educational Value:
Reinforces theory with observable outcomes
Encourages students to think critically about materials and thermal dynamics
Prepares students for lab-based assessments and practical IA design.
🧪 Activity 2: Applying the Kinetic Theory to Explain Pressure and Temperature in Gases
Curriculum Alignment:
IBDP Physics Topic B.1 – Kinetic Theory of Gases
Focuses on pressure, temperature, and the ideal gas law
Learning Outcomes:
Use the kinetic molecular theory to explain how gas temperature affects pressure
Apply the ideal gas law to real data:
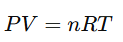
Derive conclusions about pressure-temperature relationships in closed systems
Compare theoretical and experimental results for validation
Activity Setup:
Students use a sealed syringe fitted with a pressure sensor and a thermometer to observe how heating or cooling the gas affects pressure. It’s a tangible way to see the gas laws in action.
Key Tools:
Adjustable syringe, pressure sensor, thermometer
Hot water bath and ice bath for temperature variation
Graphing software or lab notebook
Procedure:
Record baseline pressure and temperature
Heat or cool the syringe and log corresponding pressure readings
Plot pressure vs. temperature (Kelvin) to visualize the direct relationship
Compare graphs with theoretical predictions from the ideal gas law
Deep Dive Concepts:
Kinetic energy is proportional to temperature: As temperature rises, molecules move faster and exert more force on the container walls
Ideal gas behavior assumes point particles and no intermolecular forces
Reinforces the need to use Kelvin, not Celsius, in thermodynamic equations
Why Educators Love It:
Provides a real-world context for abstract gas laws
Encourages hands-on exploration of pressure systems
Offers seamless integration with discussions on scuba diving, engines, or HVAC systems.
🧠 Synthesis: Building Conceptual Bridges Between Heat and Motion
These two activities provide a powerful one-two punch in any thermal physics unit. Students begin by examining how energy flows through matter (solids vs. fluids), and then extend that learning to explore how energy drives motion in gases. Together, they help form a comprehensive understanding of energy transfer, a core theme throughout the IBDP Physics course.
Skills Strengthened:
Scientific reasoning and explanation
Real-world data collection and interpretation
Mathematical modeling and graphical analysis
Experimental design and variable control
Peer discussion and inquiry-based learning
Ideal for:
Topic B.1 instruction
Internal Assessment preparation
Formative assessments and revision labs
Bridging classical thermodynamics with molecular theory.
🔥 Turn Up the Engagement in Thermal Physics
With these ready-to-go resources, IBDP educators can turn abstract principles into memorable, engaging lab experiences that empower students to explore, question, and connect.
These aren't just worksheets—they’re conceptual gateways, designed to help learners develop scientific intuition, interpret real-world phenomena, and lay the foundation for future study in physics, engineering, or environmental science.
👉 Add these classroom-tested activities to your digital shelf today and give your students the thermal tools they need to master energy flow and motion.
